Which pH buffer solution should I use first when calibrating?
pH Buffer solutions are required to calibrate a pH controller with a pH sensor (probe). The 3 most common pH buffer solutions are pH4, pH7 and pH10, and are usually a different colour to clearly distinguish between them. pH 4 is often red, pH7 green and pH10 blue. It is recommended to write the pH value on the cap of each bottle to prevent them getting mixed up.
pH buffer solutions should always be stored in a cool dry place and should be discarded responsibly if any remains after the expiry date, which is printed on the bottle.
When calibrating a pH controller, it is best to calibrate pH7 first and then to calibrate pH4 or pH10. Typically if you are operating in the acidic range (i.e. < pH7) then you will calibrate with pH7 and then pH4. Conversely if you are operating in the basic range (i.e. > pH7) then you will calibrate with pH7 and then pH10. However, the controller slope should perfectly linear, so either is fine. However, pH7 should be calibrated first.
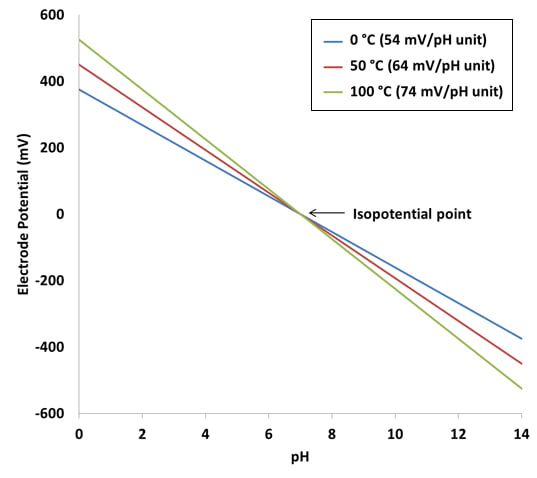
pH7 is the zero point for calibration (or first point) and pH4 or pH10 is the slope point (i.e. second point). The theoretical voltage output of a pH sensor is as follows: For every pH point increase, the mV decreases by 59mV.
pH10 = -59mV x 3 = -177mV
pH7 = 0mV
pH4 = 59mV x 3 = +177mV
As you can see the voltage output of the probe and pH10 is exactly the same as at pH4, except the polarity is different (i.e. output mV is -ve > pH7, and output mV is +ve < pH7).
When calibrating with different buffer solutions, be very careful not to contaminate the buffers. Hence, it is recommended to decant small amounts of buffers into separate cups and discard when complete. NEVER pour decanted buffer solution back into the original bottle. More about this here:
Why is it important to discard the pH buffer solution after calibration?
A recommended procedure for calibration is as follows (assuming calibrating with pH7 and then pH4):
- Rinse probe with distilled water (or pH7 buffer) and dry with paper towel.
- Decant a small amount of pH7 buffer into separate cup.
- Insert pH probe in cup, wait for reading to settle and calibrate zero point (i.e. pH7)
- Rinse probe with distilled water (or pH4 buffer) and dry with paper towel.
- Decant a small amount of pH4 buffer into a new separate cup.
- Insert pH probe in cup, wait for reading to settle and calibrate slope point (i.e. pH4)
Most controllers, no matter what variable they are measuring, are usually calibrated with a sensor at “zero point” and then at “slope point”. This means that the probe output signal is typically 0mV, 0mA or 4mA when the probe is reading the value at the zero point, and reads a high value at the slope point. pH7 probes output a theoretical 0mV at pH7 and a decent slope value at either pH4 or pH10. Hence, the pH7 should be used first and then either pH4 or pH10 next for best results
Refer to our previous blogs on pH for more information:
How to Store and Use pH Buffer Solutions for Calibrating pH